About
Juan J. Garcia Vallejo is Associate Professor and Principal Investigator at the Department of Molecular Cell Biology & Immunology. Juan has always been interested in combining his medical background with a keen interest in technology-driven biomedical research. He leads the Immune System CytOMICs research group, where he applies cutting-edge cytometry and computational methods for the characterization of the interplay between different components of the immune system and the identification of immune-based biomarkers in different disease models, including brain cancer, COVID-19, and exposure to microplastics. Juan completed an MBA in 2017 with a thesis on the management of core facilities in an academic research environment. Since 2016, he acts as Scientific Director of the Microscopy and Cytometry Core Facility of the Amsterdam UMC – Location VUmc. Juan is an active member of the International Society for the Advancement of Cytometry (ISAC) and the Core Technologies for Life Sciences (CTLS) Society.
Research Line
Characterization of the human immune system cytome (theme Tumor Immunology)
Two of the most relevant characteristics of the immune system are its highly complex heterogeneity in terms of cell types and functional states, and, the interconnectedness of all these cell subsets. Both properties are critical for the functioning of the immune system in protecting us from infections while maintaining homeostasis; but, at the same time, make this organ extremely difficult to characterize in detail. With the advent of highly multiplexed single-cell technologies we are getting closer to characterize the immune system cytome (the collection of all different cell subsets and cell states within an organ, including their interrelations) which will be critical for the identification of correlates of disease or immunological interventions as well as for elucidating the underlying mechanisms of immunity.
Two major technical advances in the field of cytometry in recent years have made possible the simultaneous characterization of more than 40 markers at a single cell level. Both mass cytometry (cytometry by time-of-flight or CyTOF) and spectral flow cytometry are allowing the routine implementation of comprehensive immunophenotyping panels in research and clinical environments. Despite of the advances, the currently available chemistries present a number of limitations. In CyTOF, the loading of metal isotopes cannot be controlled and only a few elements can be successfully loaded. In spectral flow cytometry, new fluorochromes are needed to fully exploit the configuration of current instruments. In this project, we are exploring the development of new polymer chemistries and computational methods to extend mass and spectral flow cytometry beyond the current limits of the technology and bring it to the level of targeted proteomics at the single cell level.
A functional immune atlas of glioblastoma (theme Tumor Immunology)
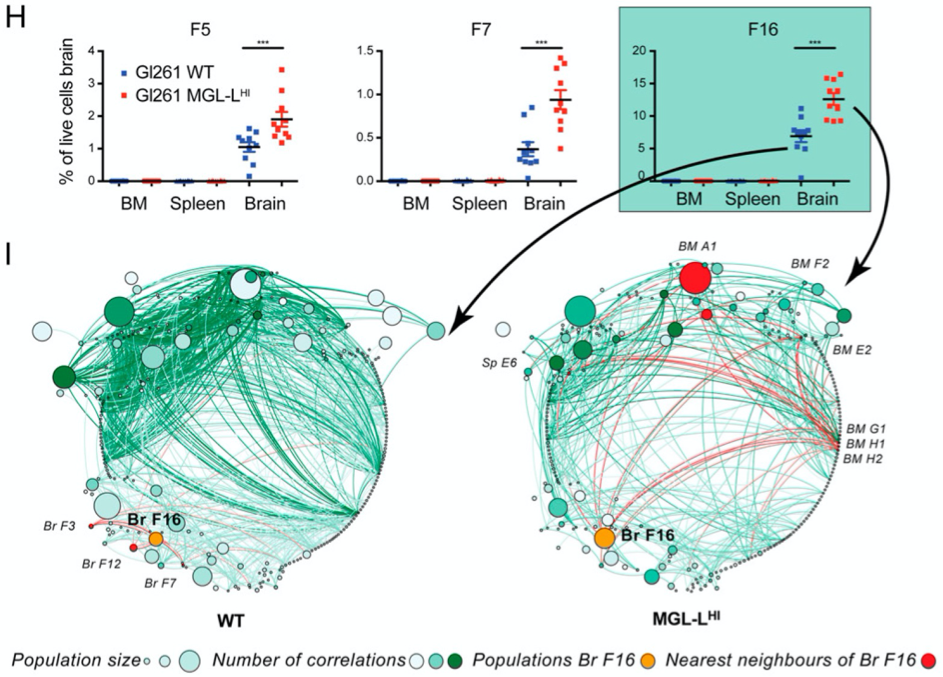
Glioblastomas are the most aggressive brain malignancies, for which immunotherapy has so far failed to prolong survival. Glioblastoma-associated immune infiltrates are dominated by tumor-associated macrophages and microglia, which are key mediators of immune suppression and resistance to immunotherapy. We have recently demonstrated that tumor-intrinsic mechanisms, such as altered glycosylation, have an impact in the distribution and phenotype of tumor-infiltrating myeloid cells. Interestingly, altered tumor glycosylation also had a distinct impact on the peripheral immune compartment, even though it is well known that glioblastomas do not metastasize. Our goal is to understand the heterogeneity of the immune infiltrate in glioblastoma with the ultimate aim to identify potential therapeutic targets. In addition, we also focus in the study of the peripheral immune system to explore the presence of signatures that can predict immune composition in the tumor and/or can have predictive/prognostic value.
Emerging research lines in the group
COVID-19 immune monitoring: The pandemic Coronavirus-disease 19 (COVID-19), caused by infection with the SARS-CoV-2 virus is characterized by a heterogeneous clinical course. While the majority of patients experience only mild symptoms, a substantial proportion develops severe disease with increasing hypoxia up to acute respiratory distress syndrome (ARDS), a disease manifestation that seems to be strongly linked to a Cytokine Release Syndrome (CRS). Importantly, severe course of COVID-19 cannot be explained only by patients’ age and concurrent diseases, because young and otherwise healthy subjects can develop lethal complications of COVID-19, suggesting that unknown susceptibility factors are involved. There is an urgent need to develop better diagnostic/prognostic tools for CRS in COVID-19 as early treatment is expected to either prevent or alleviate the severity of the ARDS. This is of special relevance while there is no readily available vaccine and, yet, more cases of COVID-19 patients are expected in successive pandemic waves of SARS-CoV-2 or, potentially, other corona viruses. In collaboration with Dr. F. van Wijk (UMCU) and Prof. S. Nezhentsev (Amsterdam UMC), we propose to use genetics and broad immunophenotyping by spectral flow cytometry to (a) characterize the immune response of COVID-19 patients at risk of developing CRS and (b) identify susceptibility factors for the development of CRS in the context of SARS-CoV-2 infection.
Microplastics and health: Our health is intrinsically related to the quality of the environment. Humans are exposed to unknown quantities of plastic particulate debris on a daily basis via air water and food. That’s why it is important to determine what adverse health outcomes may arise out of plastic particle pollution. One of the important mechanisms we think is involved in the toxicity of these particles is an interference with homeostatic immune function. This study seeks to answer the question if human blood actually contains PET used in textiles and packaging or Teflon powders extensively used in lubricants, inks and thermoplastics. And if so, what kind of hazardous immune system effects can we expect? In Collaboration with Dr. H. Leslie (VU), we are using cutting edge analytical method for the determination of microplastics in blood and, using a human blood in vitro exposure model, we seek to understand the immunological signals that show us how small plastic particles may be interfering with homeostatic immune function.
Key publications
- Crommentuijn MHW, Schetters STT, Dusoswa SA, Kruijssen LJW, Garcia-Vallejo JJ, van Kooyk Y. Immune involvement of the contralateral hemisphere in a glioblastoma mouse model. J Immunother Cancer. 2020; 8 (1): e000323.
- Dusoswa SA, Verhoeff J, (…), Garcia-Vallejo JJ. Glioblastomas exploit truncated O-linked glycans for local and distant immune modulation via the macrophage galactose-type lectin. Proc Natl Acad Sci U S A. 2020; 117 (7): 3693-3703.
- Dusoswa SA, (…), Garcia-Vallejo JJ. Glycan modification of glioblastoma-derived extracellular vesicles enhances receptor-mediated targeting of dendritic cells. J Extracell Vesicles. 2019; 8 (1): 1648995.
- Dusoswa SA, Verhoeff J, Garcia-Vallejo JJ. OMIP-054: Broad Immune Phenotyping of Innate and Adaptive Leukocytes in the Brain, Spleen, and Bone Marrow of an Orthotopic Murine Glioblastoma Model by Mass Cytometry. Cytometry A. 2019; 95 (4): 422-426.
- Duinkerken S, van Kooyk Y, Garcia-Vallejo JJ. Human cytomegalovirus-based immunotherapy to treat glioblastoma: Into the future. Oncoimmunology. 2016; 5 (9): e1214791.
Group members

Maartje Rietdijk, MSc
PhD student
My project is focused on understanding the exposure and immunotoxicity of micro- and nanoplastic (MNP) contaminants in humans by using in vitro models.

Xiangming Cai, MSc
PhD student
My work focuses on the immune monitoring of glioblastomas.