
About
Sergey Nejentsev is professor of Translational Immunology and PI in the Department of Molecular Cell Biology and Immunology (MCBI) at Amsterdam UMC, location VUmc. After graduating as pediatrician in Russia, Sergey did his PhD with Prof Jorma Ilonen in Finland and then worked as a postdoc on genetics of type 1 diabetes in Prof John Todd’s lab in Cambridge. He then started his independent research group at the department of Medicine in the University of Cambridge investigating molecular mechanisms of immune-mediated disorders, including primary immunodeficiencies and susceptibility to infection. Sergey was awarded the Royal Society University Research Fellowship and the Wellcome Trust Senior Fellowship in Basic Biomedical Science. His research was also funded by the ERC Starting and ERC Advanced grants, MRC Programme grant as well as project grants from EU FP7 and Wellcome Trust. Sergey joined MCBI in October 2018.
Research Line
Primary Immunodeficiencies
Primary Immunodeficiencies (PIDs) are a heterogeneous group of genetic disorders that affect functioning of the immune system and manifest with severe and/or recurrent infections. We identify causative mutations using whole exome or whole genome sequencing and then undertake detailed functional molecular analyses of the affected cellular pathways to uncover mechanisms leading to disease phenotypes. In this research, we closely collaborate with clinical scientists working with PID patients.
Our previous work includes the discovery of the Activated PI3K-Delta Syndrome (APDS), which is caused by a rare dominant gain-of-function mutation in phosphoinositide 3-kinase δ (Angulo et al, Science, 2013). APDS patients have antibody deficiency, suffer from recurrent respiratory infections and rapidly develop airway damage (bronchiectasis). Today, genetic analysis allows rapid diagnosis of APDS patients and they are being identified all around the world. Our findings also indicated that selective PI3Kδ inhibitors may provide a novel specific and efficient treatment for APDS, and this is now being tested in clinical trials. Furthermore, building on the findings in APDS patients we now investigate the role of human PI3Kδ in common diseases.
Our recent findings also include another novel PID, the complete RIPK1 deficiency. We showed that RIPK1-deficient immune cells have impaired proinflammatory signalling and are prone to cell death via the necroptosis pathway, which leads to dysregulated cytokine production, severe immune deficiency, arthritis and early-onset inflammatory bowel disease (Cuchet-Lourenco et al, Science, 2018). This finding highlighted the pivotal role of RIPK1 in the human immune system.
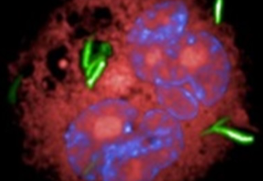
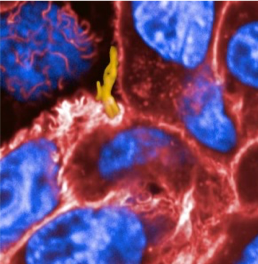
Host-pathogen interaction in tuberculosis
Tuberculosis (TB) is the leading cause of death among infectious diseases. People can be genetically predisposed to TB. Susceptibility to pulmonary TB is determined by combinations of multiple common DNA variants each having a small effect. To identify such variants, thousands of TB patients and healthy controls have to be studied. Previously, we have done a genome-wide association study in more than 11,000 subjects and identified a novel mechanism in TB mediated by the ASAP1 protein that controls dendritic cell migration (Curtis et al. Nat Genet 2015). Now we use CRISPR-Cas9 technology to further dissect mechanisms of interaction between human cells and Mycobacterium tuberculosis and develop novel host-directed therapies against TB. This research is funded by the ERC Advanced grant.
Key publications
- Cuchet-Lourenco D, Eletto D, Wu C, Plagnol V, Papapietro O, Curtis J, Ceron-Gutierrez L, Bacon CM, Hackett S, Alsaleem B, Maes M, Gaspar M, Alisaac A, Goss E, Siegmund D, Wajant H, Kumararatne D, AlZahrani MS, Arkwright PD, Abinun M, Doffinger R and Nejentsev S: Biallelic RIPK1 mutations in humans cause severe immunodeficiency, arthritis and intestinal inflammation. Science 361:810-3, 2018
- Eletto D, Burns SO, Angulo I, Plagnol V, Gilmour KG, Henriquez F, Curtis J, Gaspar M, Nowak K, Daza-Cajigal V, Kumararatne D, Doffinger R, Thrasher AJ, Nejentsev S: Biallelic JAK1 mutations in immunodeficient patient with mycobacterial infection. Nat Commun 7:13992, 2016
- Curtis J, Luo Y, Zenner HL, Cuchet-Lourenco D, Wu C, Lo K, Maes M, Alisaac A, Stebbings E, Liu JZ, Kopanitsa L, Ignatyeva O, Balabanova Y, Nikolayevskyy V, Baessmann I, Thye T, Meyer CG, Nurnberg P, Horstmann RD, Drobniewski F, Plagnol V, Barrett JC, Nejentsev S: Susceptibility to tuberculosis is associated with variants in the ASAP1 gene encoding a regulator of dendritic cell migration. Nat Genet 47:523-527, 2015
- Angulo I, Vadas O, Garçon F, Banham-Hall E, Plagnol V, Leahy TR, Baxendale H, Coulter T, Curtis J, Wu C, Blake-Palmer K, Perisic O, Smyth D, Maes M, Fiddler C, Juss J, Cilliers D, Markelj G, Chandra A, Farmer G, Kielkowska A, Clark J, Kracker S, Debré M, Picard C, Pellier I, Jabado N, Morris JA, Barcenas-Morales G, Fischer A, Stephens L, Hawkins P, Barrett JC, Abinun M, Clatworthy M, Durandy A, Doffinger R, Chilvers E, Cant AJ, Kumararatne D, Okkenhaug K, Williams RL, Condliffe A, Nejentsev S: Phosphoinositide 3-Kinase delta Gene Mutation Predisposes to Respiratory Infection and Airway Damage. Science 342:866-71, 2013
- Nejentsev S, Walker N, Riches D, Egholm M, Todd JA: Rare Variants of IFIH1, a Gene Implicated in Antiviral Responses, Protect Against Type 1 Diabetes. Science 324: 387-9, 2009
- Nejentsev S, Howson JMM, Walker NM, Szeszko JS, Field SF, Stevens HE, Reynolds P, Hardy M, King E, Masters J, Hulme J, Maier LMM, Smyth D, Bailey R, Cooper JD, Ribas G, Campbell RD, Clayton DG, Todd JA and The Wellcome Trust Case Control Consortium: Localisation of type 1 diabetes susceptibility to the MHC class I genes HLA-B and HLA-A. Nature 450: 887-92, 2007
Group members

Elham Mirfazeli, MSc
PhD student
Activated phosphoinositide 3-kinase δ in systemic lupus erythematosus

Nika Ten, MSc
PhD student
Dissecting host-pathogen interaction in tuberculosis

Olivier Papapietro, PhD
Research scientist
Host-pathogen interaction in tuberculosis

Shalmalee Kharkar, MSc
PhD student
Biomarkers of chronically hyperactivated phosphoinositide 3-kinase δ










