
About
Joke den Haan is associate professor in cellular immunology with a specific interest in the role of myeloid cells in adaptive immunity. She obtained her PhD from the University of Leiden in 1997 on the biochemical characterization of human minor histocompatibility antigens (cum laude) and did a post-doc at the University of Washington, Seattle, in the lab of Prof.dr. Mike J. Bevan on antigen cross-presentation by dendritic cell subsets (1998-2003). She joined the department of Molecular Cell Biology and Immunology in 2004 and her lab investigates the function of different types of macrophages and DCs subsets and develops cancer vaccines that specifically deliver antigens to these cell types to achieve optimal adaptive immune responses (please link to KWF movie). For these studies the group uses different types of in vitro, ex vivo, and in vivo model systems.
Research Line
Function of CD169-expressing macrophages and dendritic cells
CD169+ macrophages are localized at the marginal zone of the spleen and the subcapsular sinus of lymph nodes where they capture pathogens and extracellular vesicles. We have previously shown that antigens that are bound by splenic CD169+ macrophages are presented to B cells and transferred to cross-presenting dendritic cells that activate CD8+ and CD4+ T cell responses. We investigate the interactions of CD169+ macrophages with dendritic cells and other immune cells.
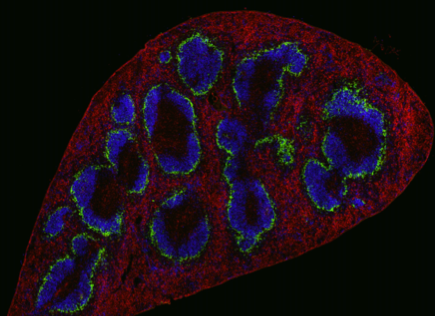
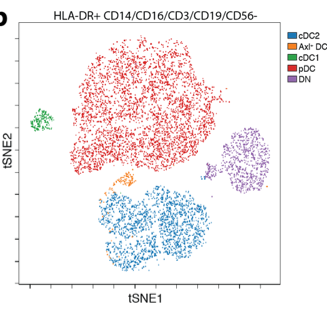
Recently, a CD169-expressing Axl+ dendritic cell subset was discovered in human PBMC. We can detect this cell type in healthy individuals and cancer patients and discovered that liposomal nanovaccines that bind to CD169 on these cells are taken up and presented to T cells.
Development of cancer nanovaccines
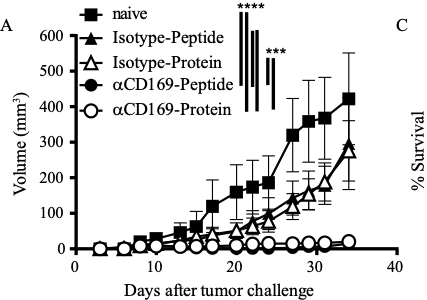
We have generated different types of nanovaccines that target cancer antigens and adjuvant specifically to CD169+ macrophages and dendritic cells in mice and men. For this we utilize CD169-specific antibodies that are conjugated to cancer antigens in the form of proteins or peptides. In addition, we have generated liposomes that contain physiological ligands for CD169 to target cancer antigens and adjuvant to CD169+ macrophages and dendritic cells. Current studies are focused on the type of adjuvant and antigen to be included for optimal immune responses. In addition, we are generating nanobodies for human CD169 and will include them in different types of nanovaccins.
Key publications
- Affandi, A.J., Grabowska, J., Olesek, K., Lopez Venegas M., Barbaria, A., Rodríguez, E., Mulder, P.P.G., Pijffers, H.J., Ambrosini, M., Kalay, H., O’Toole, T., Zwart, E.S., Kazemier, G., Nazmi, K., Bikker, F.J., Stöckl, J., van den Eertwegh, A.J.M., de Gruijl, T.D., Storm, G., van Kooyk, Y., den Haan, J.M.M. 2020.
Selective tumor antigen vaccine delivery to human CD169+ antigen presenting cells using ganglioside-liposomes. PNAS accepted. We show in this paper that liposomes that contain gangliosides can bind to human CD169+ dendritic cells and are presented to T cells. - van Dinther, D., H. Veninga, S. Iborra, E. G. F. Borg, L. Hoogterp, K. Olesek, M. R. Beijer, S. T. T. Schetters, H. Kalay, J. J. Garcia-Vallejo, K. L. Franken, L. B. Cham, K. S. Lang, Y. van Kooyk, D. Sancho, P. R. Crocker, and J. M. M. den Haan. 2018.
Functional CD169 on Macrophages Mediates Interaction with Dendritic Cells for CD8+ T Cell Cross-Priming. Cell Rep 22: 1484-1495. In this paper we describe that CD169 enables adhesion and antigen transfer to cross-presenting dendritic cells and that DNGR-1 mediated activation stimulates cross-presentation. PMID: 29425504 - Veninga, H., E. G. Borg, K. Vreeman, P. R. Taylor, H. Kalay, K. Y. van, G. Kraal, L. Martinez-Pomares, and J. M. den Haan. 2015.
Antigen targeting reveals splenic CD169 macrophages as promoters of germinal center B-cell responses.
Eur. J. Immunol 45: 747-757. In this paper we show that antigen targeting to CD169+macrophages stimulates strong germinal center B cell responses and that CD169+ macrophages retain intact antigen on their surface for two days. PMID: 25487358 - Backer, R., T. Schwandt, M. Greuter, M. Oosting, F. Jungerkes, T. Tuting, L. Boon, T. O’Toole, G. Kraal, A. Limmer, and J. M. den Haan. 2010.
Effective collaboration between marginal metallophilic macrophages and CD8+ dendritic cells in the generation of cytotoxic T cells.
Proc. Natl. Acad. Sci. U. S. A 107: 216-221. This is our first paper showing that antigens are transferred from CD169+macrophages to cross-presentation DCs for CD8+ T cell activation. PMID: 20018690 - den Haan, J. M., S. M. Lehar, and M. J. Bevan.
CD8(+) but not CD8(-) dendritic cells cross-prime cytotoxic T cells in vivo. J. Exp. Med 192: 1685-1696. This paper is the first demonstration that CD8+DCs (currently named cDC1) selectively cross-present cell-associated antigens. PMID: 11120766
Group members

Alsya Affandi, PhD
Postdoctoral researcher
My research focuses on developing nanovaccines targeting antigen-presenting-cells such as dendritic cells and macrophages, to stimulate T cell responses against cancer or pathogens. A variety of nanovaccine platforms are used including liposome and single-domain antibodies. For my research, I use spectral/imaging flow cytometry, immunoassays, microscopy, and analysis of single-cell RNA sequencing datasets.

Aru Zeling Wang, MSc
PhD student
My project is to develop a versatile cancer vaccine format in which patient-specific tumor antigens can be conjugated to antibodies specific for dendritic cells to stimulate immune responses.

Joanna Grabowska, MSc
PhD student
In my project I use CD169 targeting liposomes to efficiently deliver the antigen to splenic CD169+ macrophages. Apart from the quantitative and qualitative characterization of liposome-induced adaptive immune responses I also try to identify key mechanisms underlying these immune responses by investigating the contribution of cDC1, CD169+ macrophages and platelets. I perform in vivo immunizations and collect flow cytometry data, but also ELISA-based data and in vitro data.

Maarten Nijen Twilhaar, MSc
PhD student
I work on liposomal cancer vaccines that target to CD169-expressing macrophages, a specific subset of macrophages in the spleen. For this, we surface decorate liposomes with targeting moieties and include various antigens and adjuvants in our liposomes. Using various in vitro assays, in vivo models and multicolor flow cytometry readouts, we aim to augment the efficacy of liposomal cancer vaccination.

Rianne Bouma, MSc
PhD student
My project focuses on creating a vaccine using novel molecules targeted to CD169+ macrophages. These molecules will be conjugated to liposomes which have cancer antigen encapsulated. This will elicit a macrophage/dendritic cell mediated immune response against the specific tumor.










